
TBW : labelled water (3H2O or 2D2O) or sometimes ethanol. Since concentration = mass/ volumeVolume = mass / concentration = x/y L. measure concentration at equilibrium (y gm/L). Inject x gm of marker into compartment. Osmolalities of all body fluids are equal changes in solute concentrations are quickly followed by osmotic changes PLAYĬontinuous Mixing of Body Fluids Figure 26.3Ĭompartment Amount of Tracer Added (A) Amount of Tracer Lost From Compartment (E) Measuring Compartment Size(Law of Mass Conservation) Volume (V) Tracer Concentration (C) Amount of Tracer Remained in Compartment = A - E Compartment Volume = (A – E)/C. Plasma is the only fluid that circulates throughout the body and links external and internal environments. Nutrients, respiratory gases, and wastes move unidirectionally. Ion fluxes are restricted and move selectively by active transport. Exchanges between interstitial and intracellular fluids are complex due to the selective permeability of the cellular membranes. Net leakage of fluid from the blood is picked up by lymphatic vessels and returned to the bloodstream. Compartmental exchange is regulated by osmotic and hydrostatic pressures. = 286.5 mOsm/L 285 mOsm/L 285 mOsm/L Colloid Osmotic Pressure, Protein Osmotic Pressure, Oncotic PressureĮlectrolyte Composition of Body Fluids Figure 26.2 Body fluid compartments calculations Pc#
97% of the mass of solutes in the intracellular compartmentĬomparison of the Three Major Compartments Capillary Interstitium Intracellular Permeable to most solutes but imperm- eable to large proteins Impermeable to most solutes Na+ 140 mM Na+ 142 mM Na+ 10 mM K+ 4.5 mM K+ 4.5 mM K+ 140 mM Proteins 1.5 mM Proteins 0.1 mM Proteins 3 mM Pc = 25 mmHg P = 0 mmHg = 28 mmHg = 3 mmHg Osm. 60% of the mass of solutes in interstitial fluid. Proteins, phospholipids, cholesterol, and neutral fats account for:. Electrolytes determine the chemical and physical reactions of fluids. This reflects the activity of cellular ATP-dependent sodium-potassium pumps. Sodium and potassium concentrations in extra- and intracellular fluids are nearly opposites. Intracellular fluids have low sodium and chloride. 
Extracellular fluids are similar (except for the high protein content of plasma).Each fluid compartment of the body has a distinctive pattern of electrolytes.ĪPPROXIMATE IONIC COMPOSITION OF THE BODY WATER COMPARTMENTS Colligative Properties of Solutions: Depend on number of molecules and not on their nature. Osmolality Osmolality: millimoles of solute/Kg of H2O Osmolarity: millimoles of solute/L of H2O Activity of H2O decreases as Osmolality increases. For single charged ions, 1 mEq = 1 mOsm.mEq/L = (concentration of ion in /the atomic weight of ion) number of electrical charges on one ion.Expressed in milliequivalents per liter (mEq/L), a measure of the number of electrical charges in one liter of solution.One equivalent is equal to one-half a GMW.Divalent Ions (Ca++, Mg++, and HPO42-).Physiological Concentration: milliequivalent.Equivalent - weight of an ionic substance in grams that replaces or combines with one gram (mole) of monovalent H+ ions.Symbol “M” means moles/liter not moles.Concentration expressed as:moles per liter of solution.Osmolality is an inverse measure of water concentration.As osmolality increases, the relative number of water molecules in the solution decreases.Osmolality is the number of dissolved particles per kg of solution.Concentration expressed as:moles per kilogram of solvent.Percent Concentrations: (Solute / Solvent) x 100 Water moves according to osmotic gradients.Electrolytes have greater osmotic power than nonelectrolytes.Nonelectrolytes – examples include glucose, lipids, creatinine, and urea.Electrolytes – inorganic salts, all acids and bases, and some proteins.Other ECF – lymph, cerebrospinal fluid, eye humors, synovial fluid, serous fluid, and gastrointestinal secretions.Interstitial fluid (IF) – fluid in spaces between cells.
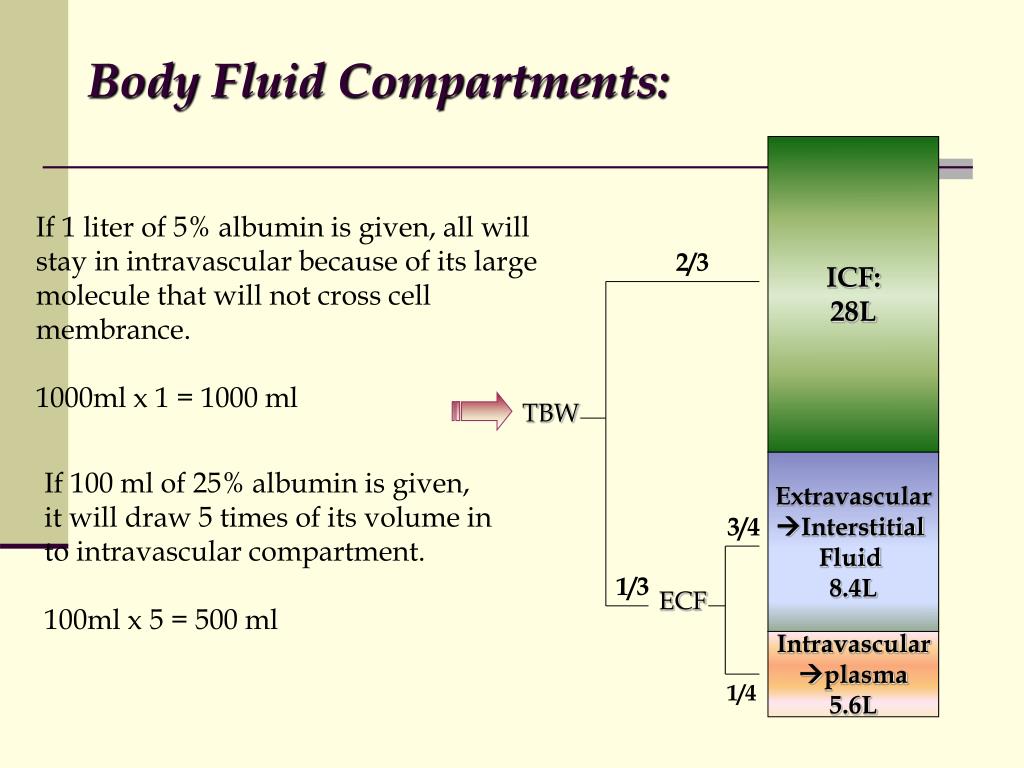 Plasma – the fluid portion of the blood. Extracellular fluid (ECF) – consists of two major subdivisions. Intracellular fluid (ICF) – about two thirds by volume, contained in cells. Water occupies two main fluid compartments. Increases in plasma osmolality trigger thirst and release of antidiuretic hormone (ADH). Metabolic water or water of oxidation (10%). Ingested fluid (60%) and solid food (30%). To remain properly hydrated, water intake must equal water output. In old age, only about 45% of body weight is water.
Plasma – the fluid portion of the blood. Extracellular fluid (ECF) – consists of two major subdivisions. Intracellular fluid (ICF) – about two thirds by volume, contained in cells. Water occupies two main fluid compartments. Increases in plasma osmolality trigger thirst and release of antidiuretic hormone (ADH). Metabolic water or water of oxidation (10%). Ingested fluid (60%) and solid food (30%). To remain properly hydrated, water intake must equal water output. In old age, only about 45% of body weight is water. 
Healthy males are about 60% water healthy females are around 50%.Total water content declines throughout life.Infants have low body fat, low bone mass, and are 73% or more water.Physiology, University of Inonu Faculty of Medicine Body Fluid Compartments & Physiological Solutions By Halil DÜZOVA, M.D.







 0 kommentar(er)
0 kommentar(er)
